PCs for GPs
Intel has been talking about its healthcare initiatives for a while, but it always knew it was reliant on regulatory approval to turn that theory into practice. Yesterday it announced that it had received clearance from the US Food and Drug Administration (FDA) on its personal health system: The Intel Health Guide.
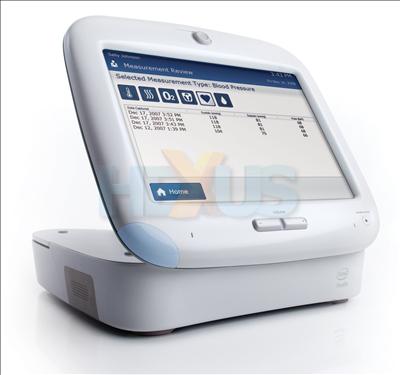
The Health Guide is a piece of technology that allows remote management of care between medical professionals and patients. It is a combination of an in-home patient device and an online interface for the healthcare professional.
“This is an important product that will improve the state and cost of health care around the world,” said Louis Burns, VP and GM of Intel’s Digital Health Group. “It results from years of research to understand the needs of the aging population and how technology can support them in their daily lives.
“With more people living with chronic diseases, we believe care can be increasingly moved outside of the hospital to the home. Through our research, we’ve learned that a home-based model of care becomes more than just delivering care to patients at home – it is about creating connections to family, friends, caregivers, and the care team.”
Intel has already completed pilot studies in the UK and US and will now step up its communication with the healthcare sector in those countries prior to an expected commercial launch in late 2008 or early 2009.
This looks like potentially quite a coup for Intel as it opens up a whole new market for it and now it has the opportunity to dominate domestic healthcare technology innovation. This is a market that’s bound to grow, especially in the West, as the average age of populations continues to increase.